Module 4: Oceanic pH Calculation
Objectives
- Calculate the carbonate species distribution given pH and CO2 (gas)
- Build a double-log plot based on a range of pHs
- Calculate the pH change and CaCO3 solubility given an increased atmospheric CO2.
- Replot other published data for comparison with our data
Overview
In this exercise, we create a simple model of the ocean, with a limited number of state variables. We examine the chemical interactions that result from atmospheric CO2 gas dissolving in water, along with calcium salts in the ocean fed by erosion of minerals on the land. The key overall process is that dissolved CO2 and Ca2+ are in equilibrium with solid CaCO3 (limestone).

Figure 1: Bleached coral (foreground) and normal coral (background). CC BY 3.0, https://en.wikipedia.org/w/index.php?curid=32829631
The major variables are given in Table 5. Given any two of the variables on the left hand column, the other four can be calculated.
| \(CO_{2 (\text{gas})}\) | \(CO_{2 (\text{dissolved})}\) |
| \(H^{+}\) | \(Ca^{2+}\) |
| \(HCO_{3}^{-}\) | \(OH^{-}\) |
| \(CO_{3}^{2-}\) | \(H_{2}CO_{3}\) |
| \(C_{T} \text{ (total dissolved inorganic carbon)}\) | \(H_{2}CO_{3}^{*}\) |
| \(\text{Alkalinity}\) |
Module resources
Download the Excel spreadsheet and R files for this module by clicking the download button in the tool bar .
Exercise 1
In the first exercise, we set pH to be 8.3 and atmospheric CO2 gas to be 3.5×10-4 atmospheres, and calculate the other variables based on these two. CO2 gas dissolves in water according to Equation (16). The dissolved CO2 easily dissociates into carbonic acid according to Equation (17). For simplicity, we will go straight from atmospheric CO2 to H2CO3*, which is the sum of dissolved CO2 and carbonic acid, using Equation (18).
\[\begin{align*} {CO}_{2(\text{gas})}&\rightleftarrows{CO}_{2(\text{dissolved})} \tag{16} \\ {CO}_{2(\text{dissolved})}+H_2O&\rightleftarrows H_2{CO}_3 \tag{17} \\ {CO}_{2(\text{gas})}+H_2O&\rightleftarrows \color{#ED7D31}{H_2CO_3^\ast} \hspace{3em} K_{H} = 3.39×10^{-2} \tag{18} \\ \end{align*}\]
The carbonic acid dissociates into a hydrogen ion (H+, or proton) and a bicarbonate ion (HCO3-), according to Equation (19). The bicarbonate further dissociates into carbonate (CO32-) and another hydrogen ion, as shown in Equation (20). The release of these two hydrogen ions is what creates the acidity of the ocean.
\[\begin{align*} \color{#ED7D31}{H_2CO_3^\ast} &\rightleftarrows \color{#00B050}{HCO_3^-}+\color{#FF0000}{H^+} \hspace{3em} K_{1} = 5×10^{-7} \tag{19} \\ \color{#00B050}{HCO_3^{-}} &\rightleftarrows \color{#5B9BD5}{{CO}_3^{2-}}+\color{#FF0000}{H^+} \hspace{3em} K_{2} = 5×10^{-11} \tag{20} \\ \end{align*}\]
Total carbon, \(C_{T}\), is defined as the sum of these carbonate species. Alkalinity is defined as the sum of the charged carbonate species and the balance of hydrogen ions and hydroxide (OH-), as shown in Equations (21) and (22). (Remember that alkalinity is the opposite of acidity.)
\[\begin{align*} C_T&=\left[\color{#00B050}{{HCO}_3^-}\right]+\left[\color{#5B9BD5}{{CO}_3^{2-}}\right]+\left[\color{#ED7D31}{H_2{CO}_3^\ast}\right] \tag{21} \\ \left[Alk\right]&=\left[\color{#00B050}{{HCO}_3^-}\right]+2\left[\color{#5B9BD5}{{CO}_3^{2-}}\right]+\left[{OH}^-\right]-\left[\color{#FF0000}{H^+}\right] \tag{22} \\ \end{align*}\]
The concentration of hydroxide can be found by rearranging Equation (23) to Equation (24) and using the dissociation constant of water, \(K_W\), and the hydrogen ion concentration.
\[\begin{align*} [OH^{-}][\color{#FF0000}{H^{+}}] &= K_{W} \hspace{3em} K_{W} = 1×10^{-14} \tag{23} \\ [OH^{-}]&=\frac{K_{W}}{[\color{#FF0000}{H^{+}]}} \tag{24} \\ \end{align*}\]
Open the Excel spreadsheet or R project and examine the tab or script Initial CO2. The chemical equations and constants are given to you already. The variables that we are forcing to be constant in Excel are
coloured grey. Enter the equations for Exercise 1 into the yellow boxes.
If we assume that calcium ion concentration does not vary much in this system, we can force it to be 5×10-4 mol L-1. Calcium carbonate (CaCO3) precipitation or dissolution is governed by the ratio (\(\mathrm{\Omega}\)) of Ca2+ concentration and CO32- concentration to the solubility constant, \(K_{sp}\), according to Equations (25) and (26). If is greater than 1, then precipitation is favourable, and conversely, if is less than 1, dissolution is favourable.
\[\begin{align*} {CO}_3^{2-} &+{Ca}^{2+}\rightleftarrows CaCO_3 \hspace{3em} K_{sp} = 1\times 10^{-8.4} \tag{25} \\ \mathrm{\Omega} &=\frac{[Ca^{2+}][CO_{3}^{2-}]}{[K_{sp\space CaCO_{3}}]} \tag{26} \\ \end{align*}\]
Have a good think about this: given these CO2 and pH, did your calculations lead to a system that favours CaCO3 dissolution or precipitation?
Exercise 2
In this exercise you will construct a double-logarithmic plot of this system, holding CO2 constant and varying the pH. Go to the second tab in Excel and copy the equations over from the first tab. Or in R, you can run a for loop to calculate the different concentrations for each of the different pHs. Then you will have some of the major variables converted to logarithms. Next you can make an x y scatter plot. If you add the data correctly, you should get a plot that looks like Figure 2.
![Double logarithmic plot of the equilibrium composition of seawater in our model [@stumm1996]](images/05-module4/picture2.png)
Figure 2: Double logarithmic plot of the equilibrium composition of seawater in our model (Stumm and Morgan 1996)
Exercise 3
This is the cool part. Here we are going to calculate a new pH given an increased atmospheric CO2 concentration. Given that we calculated the concentrations of all the other species according to the initial distribution in Exercise 1, we now hold them all constant, while increasing CO2 and calculating H+ (and therefore pH). This is not simple, because H+ concentration is tightly bound up with the concentrations of all other species, and so we cannot simply work backwards from any one equation alone. However, there is a quadratic Equation (27) that we can solve for H+ , using a fixed \(C_{T}\), rearranged to Equation (28).
\[\begin{align*} {\left[\color{#FF0000}{H^+}\right]^2C}_T &=\color{#ED7D31}{H_2{CO}_3^\ast}\left(\left[\color{#FF0000}{H^+}\right]^2+K_1\left[\color{#FF0000}{H^+}\right]+K_1K_2\right) \tag{27} \\ \left[\color{#FF0000}{H^+}\right] &=\sqrt[2]{\frac{\color{#ED7D31}{H_2{CO}_3^\ast}}{C_T}\left(\left[\color{#FF0000}{H^+}\right]^2+K_1\left[\color{#FF0000}{H^+}\right]+K_1K_2\right)} \tag{28} \\ \end{align*}\]
Solving this quadratic would be fiendishly difficult. Instead, we will use trial and error, otherwise known as our iterative solution. We will do this to find a value for H+ that is the same on both sides of the equation.
Go to the third tab and copy over all of the equations from Exercise 1. The grey boxes are the values that we will hold constant, and the first guess for H+, based on the H+ in Exercise 1. You can alter the CO2 concentration using the CO2 multiplier cell and your rapidly developing Excel and R skills. If you want to double CO2 concentration, type 2 into the multiplier cell. If you want to keep it at present levels, type 1. Repeat the calculation of Equation (28) over successive iterations, using the answer from the previous iteration as your guess for the next iteration. Eventually, the difference between your guesses will become very small and you can settle on a constant pH. (This is roughly how equilibrium-solving computer programs solve complex biogeochemical interactions, often with many more interacting variables than this.) Don’t forget that your successive iterations, are not time steps, like we had in previous weeks. Rather, they are just guesses at the right number that only exist in the model. Your final answer is the only one that has a real scientific meaning. If you want to determine the pH over a range of CO2 concentrations, you will need to repeat the calculation over ~10 rows, and save your results each time.
Big questions: if you increase CO2, does the pH rise or fall? Does CaCO3 precipitation become more or less favourable if CO2 increases?
Exercise 4
Last exercise! In this exercise, we will extract someone else’s data from their plot and make our own plot.
Go to the IPCC website and read about CO2 predictions and CaCO3 precipitation. Save their Figure 10.24 as an image file or download it here.
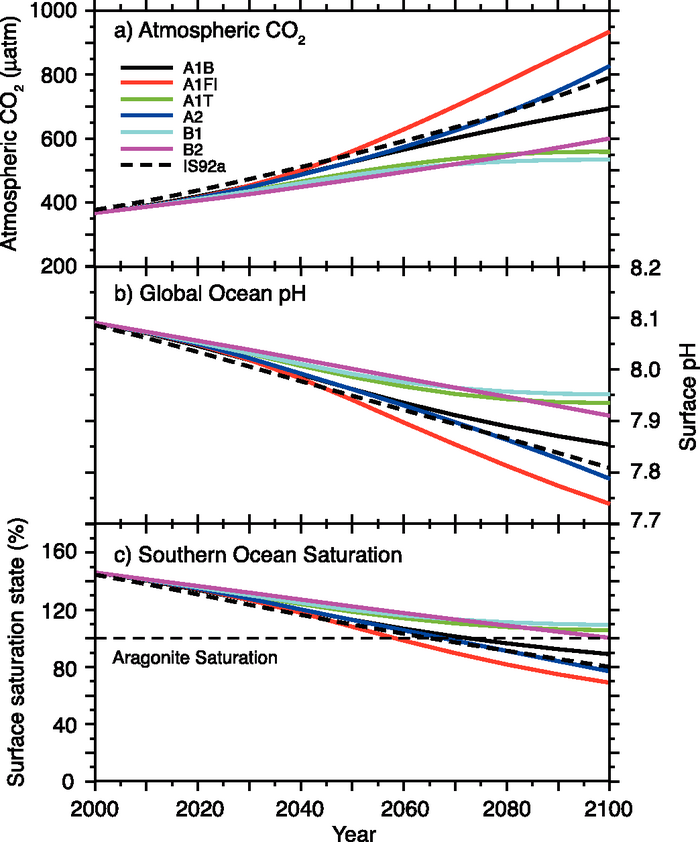
Figure 3: Changes in global average surface pH and saturation state with respect to aragonite in the Southern Ocean under various SRES scenarios.
Go to the Plot Digitizer website and download the program.
Open the IPCC image in Plot Digitizer. The program guides you through the steps you need to take: read the command at the bottom of the screen (for example, “Choose most negative end of x-axis”) and click “Done” when you have finished. It will be easiest if you make a point every 10 years. Save each dataset as a separate csv file and then combine the results in one sheet. If your x-axis points line up nicely enough, you can re-plot the data.
Compare these results with your own results. And make any plots that you feel help explain the environmental system behaviour.
Which factors cause the differences between your results and those of the IPCC models?
Submission
Submit properly formatted graphs and tables of the following sections of the lab:
- Double log plot.
- Any number of plots that compare your Exercise 3 results with the IPCC data.
The submission is to be all together in a word doc or PDF format. No screenshots of figures from Excel/Excel spreadsheets to be uploaded.
General professional formatting guidelines:
- All figures are to have adequate captions explaining them
- For graphs, figure captions go below the plot
- For tables, the caption goes above the table
- Make sure figures and their text size is readable
Excel hints:
- When there is a caption for a plot, you remove the title
- Remove the plot border and gridlines
- Make sure both axes have visible lines and tick marks
- Units need to be noted properly with the axis label - ‘Temperature (°C)’
- Round numbers to be reasonable
References
Stumm, Werner, and JJ Morgan. 1996. “Chemical Equilibria and Rates in Natural Waters.” Aquatic Chemistry 1022.