6 Inorganic Nitrogen
6.2 Overview
Nitrogen is fundamental to shaping water quality in aquatic ecosystems. Nitrogen is of particular interest in that exists in many redox states and there are a myriad of organic and inorganic reactions that make up the nitrogen biogeochemical system. NO3- and NH4+ are the primary inorganic nutrients that are taken up by aquatic plants (phytoplankton, macroalgae and macrophytes) to build biomass as organic nitrogen. As nitrogen is a core building block of an aquatic ecosystem, aed_nitrogen (\(\mathrm{NIT}\)) is designed as a low-level module for managing the inorganic nitrogen pools, and is able to be linked to by higher order modules associated with primary production, organic matter breakdown, and other ecosystem processes.
The general nitrogen redox processes specifically resolved in this module are NH4+ oxidation to NO3- by O2, and NO3- reduction to N2 with organic matter. Advanced options are available, in which the end result is generation of the nitrogen species N2O. Producing N2O uses more variables and reaction pathways and is not necessary for most nutrient cycling studies. The module also supports processes associated with the dissolved sediment flux and atmospheric deposition.
Importantly, nitrogen species react with organic matter. The description of organic matter involvement in nitrogen reactions and interactions can be found in Section (OGM). The uptake and use of these variables by phytoplankton and macroalgae are described in Section (PHY) and (MAG).
When configuring the \(\mathrm{NIT}\) module, users must first identify the level of complexity they wish to adopt in their nitrogen module configuration. Whilst the module is highly configurable, users generally adopt the default model, and if N2O or more granuallr resolution of nitrogen redox interactions are required then they can choose these more advanced options via the switch simN2O.
6.3 Model Description - Default Model
6.3.1 Process Descriptions
In the default nitrogen module configuration, the variables NO3 and NH4 are included as state variables and able to be modified by nitrification, denitrification, sediment release and atmospheric deposition; to use this basic functionality, ensure the parameter simN2O is set to 0. The main balance equations for these two simulated variables are summarised as:
where \(\mathbb{M}\) and \(\mathcal{S}\) refer to water mixing and boundary source terms, respectively, and the coloured \(\color{brown}{f}\) terms reflect the optionally configurable contributions from other modules; these include the mineralisation of \(DON\) and \(N\) cycling terms associated with photosynthesis and respiration by various biotic groups.
If users wish to include NO2 or N2O as state variables in a simulation, then refer to Section X.4
6.3.1.1 Nitrification and denitrification reactions
In an environment of high O2, NH4+ is oxidized by oxygen to NO3- via nitrification. In an environment of low O2, NO3- is reduced to N2 via the process denitrification. O2 limitation on the rate of the reaction is not included in the default setup, but can be chosen by the user, as described in the section below.
6.3.1.2 Nitrification
The rate of nitrification is assumed to vary primarily due to temperature, and also due to variation in O2 and pH, according to:
\[\begin{equation} f_{nitrf}^{NH_4} =R_{nitrf} \; \theta_{nitrif}^{T-20} \; {\underbrace{\Phi_{O2}^{nitrf}\left[O_2\right] \Phi_{pH}^{nitrf}\left[pH\right]}_{\text{Optional limitations}}} \; NH_4 \tag{6.3} \end{equation}\]where \(\theta_{nitrif}\) is a Arrhenius temperature parameter specific to nitrification, and \(T\) is the temperature produced by the host model. O2 limitation on this rate will not be included in the setup unless a link to the oxygen module is made, as described in the section below.
Oxygen limitation on nitrification: An extra option is to allow low O2 concentration to limit the nitrification rate, \(\Phi_{O_{2}}^{nitrf}\left[O_2\right]\). If the aed_oxygen module is linked to the nitrogen module, then this setting will switch on automatically. The limitation effect is calculated according to:
\[\begin{equation} \Phi_{O_{2}}^{nitrf}\left[O_2\right]=\frac{O_2}{O_2+K_{nitrif}} \tag{6.4} \end{equation}\]where \(K_{nitrif}\) is a half saturation constant concentration of oxygen. The purpose of this factor is to reduce the rate of nitrification as oxygen moves below a critical concentration.
pH limitation on nitrification: The rate of nitrification can be decreased at very high and low pH values, if pH is being simulated (see Carbon and Geochemistry). To use this function, the parameter simNitrfpH is set to .TRUE.. Around neutral pH, between the upper and lower optimum parameters, the reaction proceeds at the normal rate. Below a lower tolerance, and above an upper tolerance, the reaction is stopped entirely. Between the optimum and tolerance values, the rate is limited, in proportion to pH.
Light limitation on nitrification: This process is not currently included and is an open area for contributions.
6.3.1.3 Denitrification
Denitrification is a heterotrophic process occurring in low oxygen environments such as anoxic bottom waters and sediments. In addition to oxygen, the rate of denitrification depends on temperature and nitrate availability. It is calculated according to:
\[\begin{equation} f_{denit}^{NO_3} = R_{denit}\; \theta_{denit}^{T-20}\; \frac{NO_3}{NO_3+K_{NO3}} \; \Phi_{O2}^{denit}\left[O_2\right] NO_3 \tag{6.5} \end{equation}\]where \(R_{denit}\) is the kinetic rate constant for denitrification and \(\theta_{denit}\) is a temperature parameter specific to denitrification. The Monod term in Equation (6.5) slows the rate at low NO3- concentrations, and allows it to proceed at the maximum rate R_denit at high NO3- concentrations. The parameter KNO3 is the half-saturation parameter for limiting the reaction rate and is set by the user to slow the rate at low NO3- concentrations. This parameter should not be confused with the O2 limitation factor K_denit, mentioned below; O2 limitation on the rate of the reaction is not included in the default setup, but can be chosen by the user, as described in the section below.
Oxygen mediation of denitrification: For denitrification, oxygen slows the reaction through inclusion of an inhibition function as:
\[\begin{equation} \Phi_{O2}^{denit}\left[O_2\right]= \left\{\begin{array}{ll} \frac{K_{denit}}{O_{2}+K_{denit}},\ \ \ \ \ \ \ \Theta^{denit}_{oxylim} = 1\\ e^{(-O_{2}/K_{denit})},\ \ \ \Theta^{denit}_{oxylim} = 2\\ \end{array} \right. \tag{6.6} \end{equation}\]Such that the denitrification rate is inhibited by high O2 concentrations, using the parameter K_denit. This parameter is an O2 concentration, and should not be confused with the parameter KNO3 which is a NO3- concentration. To use the process shown in Equation (6.6), the switch oxy_lim, \(\Theta_{oxylim}^{denit}\), set to the number 1.
Alternatively, the rate of denitrification can be set to decreases exponentially at high O2 concentrations, also using the parameter Kdenit. This is the same parameter as for the inhibition function described above, but applied differently.
6.3.1.4 Atmospheric deposition
Nitrogen can be added to the surface cells of an aquatic system through two kinds of atmospheric deposition: wet and dry. These functions can be turned on by setting the parameters simDryDeposition and simWetDeposition to .TRUE..
\[\begin{equation} f_{atmdep}^{NOx}=\left[{\underbrace{F_{atm}^{DIN}}_{\text{Dry flux}}}+{\underbrace{R_n\left[t\right] {DIN}_{atm}}_{\text{Wet flux}}}\right] \frac{{k}_{DINdepNO_x}}{\Delta z_{surf}} \tag{6.7} \end{equation}\] \[\begin{equation} f_{atmdep}^{NH_4}=\left[{\underbrace{F_{atm}^{DIN}}_{\text{Dry flux}}} + {\underbrace{R_n\left[t\right] {DIN}_{atm}}_{\text{Wet flux}}}\right]\frac{\left({1-k}_{DINdepNO_x}\right)}{\Delta z_{surf}} \tag{6.8} \end{equation}\]
where \(\Delta z_{surf}\) is the surface layer thickness. Wet deposition adds nitrogen as a result of rain, \(R_n\) (m/day), which is a variable or a boundary condition provided as an environment variable from the host model. The deposition from rain is proportional to the atmospheric nitrogen concentration atm_din_conc. As with dry deposition, the proportion that is assigned to NOx and NH4+ is given by the same parameter f_dindep_nox.
6.3.1.5 Sediment flux
NO3- and NH4+ can flux into the water column from the sediment, using the parameters \(F_{sed}^{nit}\) and \(F_{sed}^{amm}\), which set the maximum flux rate. The maximum flux rate is limited by temperature, using the parameters theta_sed_nit and theta_sed_amm, according to
\[\begin{equation} f_{sed}^{NOx}=F_{sed}^{nit} \theta_{sednit}^{T-20}\Phi_{O2}^{nit}\left[O_2\right] \tag{6.9} \end{equation}\] \[\begin{equation} f_{sed}^{NH4}=F_{sed}^{amm} \theta_{sedamm}^{T-20}\Phi_{O2}^{amm}\left[O_2\right] \tag{6.10} \end{equation}\]
This assumes that there is a higher flux at higher temperatures; set \(\theta_{sed}=1.0\) to remove any temperature dependance.
Oxygen mediation of the sediment nitrogen fluxes: As with the nitrification reaction, the sediment flux can be changed by bottom water O2 concentration. If the aed2_oxygen module is correctly linked to the nitrogen module then this setting will switch on automatically. At low O2 concentrations, the amount of NO3- fluxing out of the sediment is decreased and at high O2 concentrations, it is set close to the F_sed_nit, as shown in Equation (6.11). This is a convenient simplification that can be tuned within this module, rather than a more complicated full set of biogeochemical reactions. The parameter K_sed_nit can be used to tune the NO3- flux dependence on bottom water O2. A similar function can be used to tune the NH4+ flux using the parameter K_sed_amm, as shown in Equation 13. At high O2 concentrations, NH4+ flux decreases, and at low O2 concentrations, the flux is close to the parameter F_{sed}^{amm}.
Advanced options: The approach described here is the most simple and default method for capturing DIN fluxes from the sediment, and is sometimes referred to the static model. This approach can be extended to allow for spatial variability in \(F_{sed}^{nit}\) and \(F_{sed}^{amm}\), by engaging the link to the aed_sedflux module, where the host models support multiple benthic cells or zones. In this case, users input spatially discrete values of \(F_{sed}^{nit}\) and \(F_{sed}^{amm}\).
Where dynamic rates of DIN species are required to flux to/from the sediment (e.g. in response to episodic loading of organic material to the sediment, or for assessment of long-term changes in N loading), then the above expressions (Eq (6.9)-(6.12)) are replaced instead with dynamically calculated variables in aed_seddiagenesis, via a link created with the aed_sedflux module.
6.3.2 Variable Summary
The default variables created by this module, and the optionally required linked variables needed for full functionality of this module are summarised in Table X.
6.3.3 Parameter Summary
The default parameters and settings used by this module are summarised in Table @ref(tab:nitrogen_tab1).
| Parameter name | Description | Unit | Parameter type | Default | Typical range |
|---|---|---|---|---|---|
| Nitrification/Denitrification | |||||
|
Kinetic rate constant for nitrification |
day-1 |
real |
0.01 |
0.001-0.2 |
|
Temperature dependence of nitrification |
real |
1 |
1.05-1.12 | |
|
Kinetic rate constant for denitrification |
day-1 |
real |
0.01 |
|
|
Temperature dependence of denitrification |
Unitless |
real |
1 |
|
|
Half saturation constant for NO3- limitation on denitrification |
mmol m-3 |
real |
5 |
|
|
O2 inhibition of denitirification or exponential reduction |
mmol m-3 |
real |
150 |
|
|
Integer |
1 |
1 or 2 | ||
|
Minimum tolerance |
pH unit |
real |
5.5 |
|
|
Minimum optimum |
pH unit |
real |
7.1 |
|
|
Maximum optimum |
pH unit |
real |
7.9 |
|
|
Maximum tolerance |
pH unit |
real |
9 |
|
| Atmospheric Deposition | |||||
|
Switch to turn on atmospheric deposition |
LOGICAL |
TRUE |
||
|
Switch to turn on rain deposition |
LOGICAL |
TRUE |
||
|
Atmospheric DIN deposition rate |
mmol m-2 d-1 |
real |
0 |
|
|
Atmospheric DIN composition |
mmol m-3 |
real |
0 |
|
|
Fraction of DIN that is NOx |
real |
0.5 |
||
| Sediment Flux | |||||
|
Sediment NO3- flux |
||||
|
Sediment NH4+ flux |
||||
|
Sediment NO3- flux |
mmol m-2 d-1 |
real |
30 |
|
|
Sediment NH4+ flux |
mmol m-2 d-1 |
real |
30 |
|
|
Temperature dependence on sediment NO3- flux |
real |
1 |
||
|
Temperature dependence on sediment NH4+ flux |
real |
1 |
||
6.3.4 Optional Module Links
Other nitrogen sources/sinks are indicated in Eq (6.1)-(6.2). These include:
- Ammonium release during bacterial mineralisation of DON in the organic matter module - aed_organic_matter;
- Nitrate consumption during denitrification of DON in the organic matter module - done in aed_organic_matter if simDenitrification == 1;
- Oxygen consumption during nitrification is done in the nitrogen module - [aed_nitrogen][Inorganic Nutrients: C/N/P/Si];
- Ammonium and nitrate uptake by phytoplankton phtosynthesis is done in the phytoplankton module - aed_phytoplankton;
- Ammonium and nitrate uptake by macroalgae phtosynthesis is done in the macroalgae module - aed_phytoplankton;
- Ammonium and nitrate release and/or consumption from the sediment can be managed via the sediment flux module - aed_sedflux;
- Ammonium and nitrate release and/or consumption from the sediment can be computed dynamically using the sediment diagnenesis module - aed_seddiagnesis;
6.4 Model Description - Adding N2O Reactions
The basic function of the nitrogen model is described above, when the parameter simN2O is set to the number 0. Users may extend their model to simulate the N cycle in more detail, including resolution of intermediate N pools. Using these advanced options, there are extra reaction pathways that are not critical for resolving basic nutrient cycling processes, but are needed for calculating the concentration of the greenhouse gas N2O.
Here, these advanced module options are outlined, for when the user sets the parameter simN2O to the number 1 or 2. Simulation option 1 includes N2O, using a relatively simplistic assumption that N2O passively “leaks out” as a by-product or is consumed during the traditional nitrification-denitrification reactions. Simulation option 2 is a more comprehensive biogeochemical depiction of N cycling between its different redox states and considering the full diversity of autotrophic and heterotrophic pathways.
In addition to different N reaction pathways in these sub-models, the inclusion of additional inorganic N components is also accompanied by new process pathways for atmospheric exchange and sediment interaction.
6.4.1 Process Descriptions (simN20 = 1)
The simN2O 1 option is based on the conceptual model from Babbin et al. or Nevison. If the parameter simN2O is set to the number 1, N2O is both created and consumed through the denitrification reaction, and produced during nitrification; these rates are oxygen dependent.
The main balance equation for the additional N2O is summarised as:
\[\begin{equation} \frac{\partial N_{2}O}{\partial t} = {f_{nitp}^{N_{2}O}} + {f_{denp}^{N_{2}O}} - {f_{denc}^{N_{2}O}} + \color{#394E73}{f_{atm}^{N_{2}O}} + \color{#8B701F}{f_{sed}^{N2O}} \tag{6.13} \end{equation}\]6.4.1.1 Denitrification production of N2O
All of the NO3- consumed by denitrification is assumed to first produces N2O. The rate of production of N2O is multiplied by half because there is one mole of N2O produced for every two moles of NO3- consumed. The rate of denitrification is calculated as described here, and can have different O2 limitations, using the parameter use_oxy.
6.4.1.2 Denitrification consumption of N2O
Denitrification can also consume N2O, when the N2O acts as a high energy yielding oxidant that reacts with organic matter. The organic matter reaction is described in the organic matter model page here. The rate of N2O consumption is calculated as shown in Equation (6.15). Rn2o is a kinetic rate constant that sets the speed of the reaction and Kn2oc is the parameter for the exponential O2 inhibition of the reaction.
6.4.1.4 Atmospheric N2O Flux
N2O fluxes from the atmosphere are calculated from the difference between water and atmospheric concentration:
\begin{equation} f_{atm}^{N_{2}O}=k_{N_{2}O}(N_{2}O-N_{2}O_{atm}) \end{equation}
Where \(N_{2}O_{atm}\) is the user defined atmospheric value, and \(k_{N_{2}O}\) is the piston velocity; refer to generic utility Section X for more detail using aed2_gas_piston_velocity, and the n2o_piston_model options.
6.4.1.5 Sediment N2O Flux
N2O flux from the sediment is calculated in a similar way to the fluxes of NO3- and NH4+(Equation 22).
\begin{equation} \(f_{sed}^{N2O}=F_{sed}^{n2o} \theta_{sedamm}^{T-20}\Phi_{O2}^{n2o}\left[O_2\right]\) \end{equation}
\[ f_{sed}^{N_{2}O}=F_{sed}^{N_{2}O}\theta_{sedamm}^{T-20}\Phi_{O_{2}}^{N_{2}O}[O_2] \] {#eq:label}
6.4.2 Process Descriptions (simN2O = 2)
The simN2O 2 option uses the terminology from Weissbach et al. (2017) for the names of the reaction processes, in order to avoid the ambiguity found across publications. The model processes are described as being part of either the oxidation pathway or reduction pathway.
The main balance equations for the additional simulated variables are summarised as:
\[\begin{equation} \frac{\partial{NO}_3}{\partial t}=f_{Nitratation}-f_{Denitratation}+f_{atmdep}^{NOx}+f_{sed}^{NO3} \end{equation}\] \[\begin{equation} \frac{\partial{NO}_2}{\partial t}={f_{Nitritation}+f}_{Denitratation}-f_{Denitritation}+f_{sed}^{NO2} \end{equation}\] \[\begin{equation} \frac{\partial N_2O}{\partial t}=f_{Nitrousation}+f_{nitrous\ denitritation}-f_{denitrousation}+f_{atm}^{N2O}+f_{sed}^{N2O} \end{equation}\] \[\begin{equation} \frac{\partial{NH}_4}{\partial t}=f_{DNRA}+f_{NH4\ release}-f_{NH4\ \ oxidation}+f_{atmdep}^{NH4}+f_{sed}^{NH4} \end{equation}\]
6.4.2.1 Nitrogen Oxidation Pathways
In the oxidation pathway, reduced N in the form of ammonium is first oxidised to NO2 and N2O before NO3, as seen in Figure 1. These processes are described next.
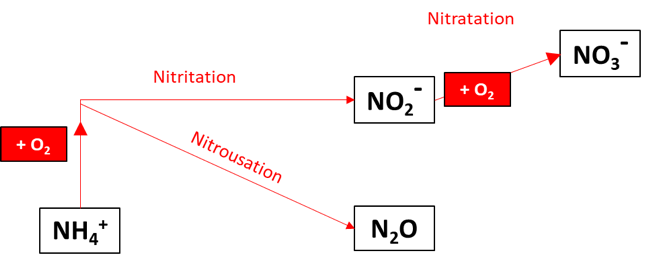
Figure 6.1: Nitrogen oxidation pathways resolved when simN2O=2.
6.4.2.2 Nitratation
Nitratation is the production of NO3^- (nitrate) from NO2- by O2 oxidation. The rate is calculated as a second-order reaction proportional to both NO2- and O2 concentrations (Equation 14). The parameter Rno2o2 is the kinetic rate constant for the reaction.
6.4.2.3 Ammonium oxidation
NH4+ is oxidized by O2 to both N2O and NO2-. The rate of NH4+ consumption is the second-order reaction proportional to both O2 and NH4+ concentrations (Equation 15). The parameter Rnh4o2 is the kinetic rate constant for the reaction.
6.4.2.4 Nitritation
Nitritation is the production of NO2- (nitrite) under conditions of high O2 concentration, from ammonium oxidation. It is limited by a partitioning function, in which the constant Kpart_ammox is the same as that for nitrousation, described below. Above a critical O2 concentration, this reaction pathway is favoured (Equation 16). The term R~Ammonium oxidation~ refers to the rate equation described above, rather than a kinetic constant.
6.4.2.5 Nitrousation
Nitrousation is one of two pathways that produce N2O using simN2O set to the number 2 (the other pathway is the reduction of NO2- via nitrous denitritation). This process occurs under conditions of low O2 concentration, and it is inhibited by oxygen, using an inhibition function (Equation 18). The constantKpart_ammox is the same as that in the nitritation process, which means that below a critical O2 concentration, this reaction pathway is favoured over nitritation. The reaction is multiplied by half, because for each mole of NH4+ oxidized, one mole of N2O is produced. The term R~Ammonium oxidation~ refers to the rate equation described above, rather than a kinetic constant.
6.4.2.6 Organic matter oxidation - nitrogen reduction reactions
When the simN2O switch is set to 2, the complex set of N2O reactions is used. The details of the organic matter processes are explained on the organic matter model page here. The five nitrogen processes are explained here.
6.4.2.7 Deammonification (anammox) pathway
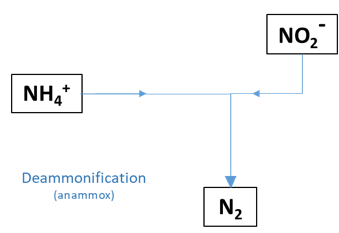
Figure 6.2: Anammox pathways.
The deammonification pathway is the oxidation of NH4+ by NO2- to produce N2. It uses neither organic matter nor O2, although it is inhibited by the presence of O2. This process is sometimes referred to as anammox (anaerobic ammonium oxidation), however, Weissbach et al (2017) use the term deammonification to distinguish it from the anammox commercial bioreactor. The parameter Rnh4no2 is the kinetic rate constant for this reaction. The parameter Kin_deamm is the O2 concentration parameter for O2 inhibition of this reaction.
6.4.3 Variable Summary
6.4.3.1 State variables
The following state variables are relevant to the nitrogen module, and dependent variables from other modules (Table x).
| Symbol | Variable | AED identifier | Unit | Comment |
|---|---|---|---|---|
| aed_nitrogen | ||||
NO3- |
Nitrate |
|
mmol m-3 |
simN2O=1,2 |
NO2- |
Nitrite |
|
mmol m-3 |
simN2O=2 only |
N2O |
Nitrous oxide |
|
mmol m-3 |
simN2O=1,2 |
NH4+ |
Ammonium |
|
mmol m-3 |
simN2O=1,2 |
N2 |
Dinitrogen |
|
mmol m-3 |
not tracked |
| Dependent variables | ||||
O2 |
Oxygen |
|
mmol m-3 |
|
DOC |
Dissolved organic carbon |
|
mmol m-3 |
|
6.4.4 Parameter summary
An overview of the parameters used in the nitrogen module for users selecting simN2O 1 or 2, are summarised in Table X.
| Parameter name | Description | Unit | Parameter type | Default | Typical range | Comment |
|---|---|---|---|---|---|---|
|
Switch to use simple or advanced N reactions |
INTEGER |
1 |
0, 1, 2 |
||
|
Kinetic rate constant for N2O consumption |
s-1 |
AED_REAL |
0.0015 |
||
|
Exponential O2 inhibiting parameter for N2O consumption through denitrification |
mmol m-3 |
AED_REAL |
0.3 |
||
|
O2 threshold for switching on N2O production from nitrification |
mmol m-3 |
AED_REAL |
3 |
||
|
O2 inhibition parameter |
mmol m-3 |
AED_REAL |
0.26 |
||
|
O2 inhibition parameter |
AED_REAL |
-0.0006 |
|||
|
Switch to use simple or advanced N reactions |
INTEGER |
2 |
0, 1, 2 |
||
|
Kinetic rate constant for nitratation |
mmol-1 m3 s-1 |
AED_REAL |
1 |
||
|
Kinetic rate constant for nitratation |
mmol-1 m3 s-1 |
AED_REAL |
1 |
||
|
Partitioning parameter for the products of ammonium oxidation, based on O2 concentration |
mmol m-3 |
AED_REAL |
20 |
||
|
Kinetic rate constant for DOC mineralisation |
mmol-1 m3 s-1 |
AED_REAL |
0.001 |
OGM | |
|
O2 concentration for inhibition and limitation |
mmol m-3 |
AED_REAL |
0.886 |
OGM | |
|
Temperature parameter |
AED_REAL |
1 |
OGM | ||
|
1 |
OGM | ||||
`Klim_denitrousv |
N2O concentration to limit denitrousation and to inhibit denitratation and denitritation |
mmol m-3 |
AED_REAL |
1 |
||
|
O2 concentration to inhibit denitrousation |
mmol m-3 |
AED_REAL |
0.205 |
||
|
NO3- concentration to limit denitratation |
mmol m-3 |
AED_REAL |
1 |
||
|
O2 concentration for inhibiting denitratation |
mmol m-3 |
AED_REAL |
20 |
||
|
NO2- concentration parameter that inhibits denitratation and limits denitritation |
mmol m-3 |
AED_REAL |
1 |
||
|
Partitioning parameter for the products of denitritation |
mmol m-3 |
AED_REAL |
1 |
||
|
Switch to use simple or advanced N reactions |
INTEGER |
2 |
0, 1, 2 |
||
|
Kinetic rate constant for deammonification |
mmol-1 m3 s-1 |
AED_REAL |
1 |
||
|
O2 concentration for inhibition of deammonification |
mmol m-3 |
AED_REAL |
0.886 |
||
|
Switch to use simple or advanced N reactions |
INTEGER |
1 or 2 |
0, 1, 2 |
||
|
Atmospheric N2O concentration |
mmol m-3 or ppm |
AED_REAL |
0.32×10-6 |
||
|
Switch to use simple or advanced N reactions |
INTEGER |
1 or 2 |
0, 1, 2 |
||
|
||||||
|
Maximum N2O sediment flux rate |
mmol m-2 y-1 |
0 |
|||
|
O2 inhibition concentration parameter |
mmol m-2 y-1 |
AED_REAL |
30 |
||
|
Temperature limiting factor for sediment N2O flux |
AED_REAL |
1 |
6.4.5 Optional Module Links
As with the default model, other nitrogen sources/sinks are indicated in Eq s… . These include:
- Ammonium release during bacterial mineralisation of DON in the organic matter module - aed_organic_matter;
- Nitrate consumption during denitrification of DON in the organic matter module - done in aed_organic_matter if simDenitrification == 1;
- Oxygen consumption during nitrification is done in the nitrogen module - [aed_nitrogen][Inorganic Nutrients: C/N/P/Si];
- Ammonium and nitrate uptake by phytoplankton phtosynthesis is done in the phytoplankton module - aed_phytoplankton;
- Ammonium and nitrate uptake by macroalgae phtosynthesis is done in the macroalgae module - aed_phytoplankton;
- Ammonium and nitrate release and/or consumption from the sediment can be managed via the sediment flux module - aed_sedflux;
- Ammonium and nitrate release and/or consumption from the sediment can be computed dynamically using the sediment diagnenesis module - aed_seddiagnesis;
6.5 Setup & Configuration
An example aed.nml parameter specification block for the aed_nitrogen module is shown below:
&aed_nitrogen
!-- Initial values
amm_initial = 2.6
nit_initial = 0.1
n2o_initial = 0.1
!-- Nitrification
Rnitrif = 0.01358676
Knitrif = 62.02209
theta_nitrif = 1.08
nitrif_reactant_variable = 'OXY_oxy'
nitrif_ph_variable = ''
simNitrfpH = .false.
Rnh4o2 = 1.0
Rno2o2 = 1.0
!-- N2O reactions
simN2O = 0
Rn2o = 0.05
Kpart_ammox = 1.0
Kin_deamm = 1.0
atm_n2o = 3.2e-07
n2o_piston_model = 4
!-- Annamox
Rnh4no2 = 0.001
kanammox = 0.001
Kanmx_nit = 1.320344
Kanmx_amm = 0.8666655
!-- De-nitrification
Rdenit = 9.968717
Kdenit = 29.86566
theta_denit = 1.062862
Rdnra = 0.01123021
Kdnra_oxy = 0.360534
!-- Atmospheric deposition
simDryDeposition = .true.
atm_din_dd = 0.5
simWetDeposition = .true.
atm_din_conc = 0.5
!-- Sediment fluxes
Ksed_amm = 41.25
Ksed_nit = 73.26015
Fsed_n2o = 0
Ksed_n2o = 100
theta_sed_amm = 1.068994
theta_sed_nit = 1.068994
Fsed_amm = 1.0
Fsed_nit = -0.05
! Fsed_amm_variable = 'SDF_Fsed_amm'
! Fsed_nit_variable = 'SDF_Fsed_nit'
/6.6 Case Studies & Examples
6.6.1 Case Study: Yarra River Estuary
Bruce et al. (2015) demonstrate the base nitrogen setup in a 3D model of Yarra River estuary, an urban riverine estuary in Melbourne, Australia, that experiences dynamic movement of a hypoxic salt-wedge based on river flow and tidal interactions.
The results of the simulation are shown in Figure X, highlighting the change in surface and bottom NH4, NO3 and organic nitrogen pools along the estuary length.
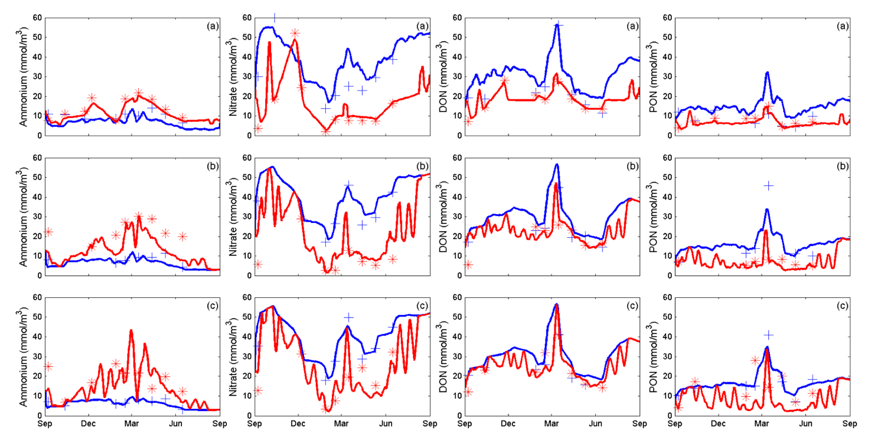
Figure 6.3: Time series data for surface (blue) and bottom (red) ammonium, nitrate, dissolved and particulate organic nitrogen for (a) Morell Bridge, (b) Scotch College, (c) Bridge Road. Crosses and asterisks represent observed data for surface and bottom concentrations respectively and solid lines simulated data.
6.6.2 Publications
| Author/Year | Paper Title | Description |
|---|---|---|
Bruce et al. (2015) |
A model of oxygen and nitrogen biogeochemical response to hydrodynamic regimes in the Yarra River estuary |
NA |
Huang et al. (2019) |
An integrated modelling system for water quality forecasting in an urban eutrophic estuary: The Swan-Canning Estuary virtual observatory |
NA |